Background and Overview
Tildipirosin is a novel animal-specific sixteen-membered ring macrolide semi-synthetic antibiotic, originally developed by Intervet International BV. It is a derivative of tylosin. On March 8, 2011, Intervet International BV in the Netherlands obtained approval from the European Medicines Agency’s Committee for Medicinal Products for Veterinary Use (CVMP) for a sterile injectable solution containing tildipirosin as the main ingredient (trade name: Zuprevo), which was subsequently approved for marketing in EU countries. Tildipirosin is a broad-spectrum antibacterial drug with strong antibacterial activity against some Gram-positive and Gram-negative bacteria, and it is particularly sensitive to pathogens causing respiratory diseases in pigs and cattle. It also has advantages such as being animal-specific, requiring low dosage, providing full treatment with a single administration, having an ultra-long elimination half-life, high bioavailability, low residue, and safe use.
Preparation
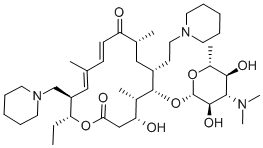
A method for synthesizing tildipirosin, characterized by the following process steps:
- Dissolving tylosin tartrate in water, heating to 53-57°C, then adjusting the pH of the solution to 0.5-1.0 with hydrobromic acid, and reacting with heat preservation to obtain an aqueous solution A of 23-hydroxy-20-oxo-5-O-mycaminosyl-tylonolide;
- Adding dichloromethane to the aqueous solution A obtained in step 1), adjusting the pH to 9.0-9.5 with alkali, stirring evenly, allowing to stand for layering, and obtaining a dichloromethane solution B of 23-hydroxy-20-oxo-5-O-mycaminosyl-tylonolide;
- Dissolving KBr solid in water, adding NaClO, stirring to dissolve, and then adjusting the pH to 8.5-9.0 with saturated NaHCO3 to obtain solution E;
- Adding 2,2,6,6-tetramethylpiperidine-1-oxyl to the dichloromethane solution B obtained in step 2) and dissolving it, then cooling to 0-5°C, slowly adding solution E dropwise until the solution turns orange-yellow, stopping the addition, reacting with heat preservation for 2-3 hours, washing with purified water, retaining the organic phase to obtain a dichloromethane solution F of 20,23-dioxo-5-O-mycaminosyl-tylonolide;
- Adding excess piperidine to solution F, heating to 40-45°C, then adding formic acid for catalytic reaction, reacting under reflux with heat preservation for 1-1.5 hours, evaporating under reduced pressure until the solution becomes viscous, slowly cooling to 0-5°C, crystallizing, and filtering to obtain tildipirosin crude product G.
Pharmacological Action
The antibacterial action of tildipirosin is similar to that of tylosin, exhibiting strong inhibitory effects against Gram-positive bacteria and some Gram-negative bacteria. It is particularly sensitive to pathogens causing respiratory diseases in pigs and cattle, such as Pasteurella multocida, Actinobacillus pleuropneumoniae, Bordetella bronchiseptica, Haemophilus parasuis, Mannheimia haemolytica, Histophilus somni, Mycoplasma, Spirochetes, and Brucella. The mechanism of action of tildipirosin, similar to other macrolide drugs like tylosin, involves binding to the 50S subunit of sensitive bacteria’s ribosomes, inhibiting and preventing the synthesis and elongation of polypeptide chains, thereby affecting bacterial protein synthesis. However, it is important to note that due to the presence of three basic amino groups in tildipirosin, it can form different charged forms under different pH conditions. The amount of charge is a key factor in disrupting bacterial lipid solubility and penetrating the outer membrane of Gram-negative bacteria. Therefore, the in vitro antibacterial activity of tildipirosin is significantly affected by pH; under acidic conditions, the amino groups are protonated, leading to a decrease in antibacterial activity; under alkaline conditions, it exhibits higher antibacterial activity. According to reports, the in vitro antibacterial activity of tildipirosin, compared with tylosin and tilmicosin, shows better antibacterial effects against Gram-negative bacteria such as Pasteurella multocida and Actinobacillus pleuropneumoniae. However, its inhibitory effect on Actinobacillus pleuropneumoniae is not as strong as that of tylosin and tilmicosin.
Applications
Currently, there are reports from abroad regarding the efficacy of tildipirosin in preventing and treating respiratory diseases in pigs and cattle. Miyake et al. compared the antibacterial activity of tildipirosin and tilmicosin against Pasteurella haemolytica using the broth microdilution method. The experimental results showed that the antibacterial activity of tildipirosin was significantly better than that of tilmicosin. At the same time, they also compared the therapeutic effects of tildipirosin and tilmicosin on cattle with respiratory diseases. The cattle in the tilmicosin treatment group were administered the drug subcutaneously at a daily dose of 10mg/kg body weight, while the cattle in the tildipirosin treatment group were administered a single subcutaneous dose of 4mg/kg body weight. After 5 days, the mortality and recovery rates of the two treatment groups were statistically analyzed, and bacteria were isolated and identified. The results showed that the recovery rate in the tildipirosin group was higher than that in the tilmicosin group.
Pasteurella multocida and Mannheimia haemolytica were not isolated from the cattle in the tildipirosin treatment group, while they could be isolated from the tilmicosin treatment group. This indicates that the therapeutic effect of tildipirosin is better than that of tilmicosin, and tildipirosin also has better preventive and therapeutic effects on fever symptoms during animal transportation. Regarding the therapeutic effect of tildipirosin on Mannheimia haemolytica, a pathogenic bacterium of bovine respiratory infections, a single subcutaneous dose of 4mg/kg body weight resulted in Mannheimia haemolytica levels below the limit of quantification (LOQ = 10 CFU/g) in all collected samples. Intervet compared the recovery rates of tildipirosin (4mg/kg body weight single intramuscular injection) and florfenicol in treating pigs with respiratory system diseases. The recovery rate of tildipirosin (86%) was higher than that of florfenicol (81%). They also compared the recovery rates of tildipirosin (4mg/kg body weight single intramuscular injection) and oxytetracycline in treating pigs with respiratory system diseases. The recovery rates were similar for both groups, 93% for tildipirosin and 92% for oxytetracycline, and there was no significant difference in weight gain between the two treatment groups.
Rohdich et al. compared the recovery and mortality rates of tildipirosin (4mg/kg body weight single subcutaneous injection) and oxytetracycline (2.5mg/kg body weight single subcutaneous injection) in treating cattle with respiratory system diseases. The recovery rate of tildipirosin (84.8%) was significantly higher than that of oxytetracycline (79.3%). There were no deaths in the tildipirosin group, while the mortality rate in the oxytetracycline treatment group was 0.6%, indicating that tildipirosin is significantly more effective than oxytetracycline in treating bovine respiratory system diseases. Secretariat et al. compared the recovery rates of tildipirosin and tylosin in treating cattle with respiratory diseases. The results showed that the recovery rate of tildipirosin was 84.4%, while that of tylosin was only 79.3%, indicating that tildipirosin is also more effective than tylosin in treating bovine respiratory system diseases.