Background and Overview
Enrofloxacin is an off-white crystalline powder, very slightly soluble in water and alcohol, and freely soluble in acetic acid, hydrochloric acid, or sodium hydroxide solutions. It is a synthetic third-generation fluoroquinolone antibacterial drug, also known as ethylciprofloxacin or enrofloxacin. It was approved by the FDA on October 4, 1996, and is a fluoroquinolone antibacterial drug specifically for livestock, poultry, and aquaculture. It exhibits broad-spectrum antibacterial activity and strong permeability, with a potent bactericidal effect against Gram-negative bacteria. It also demonstrates good antibacterial activity against Gram-positive bacteria and mycoplasma. It is well absorbed orally, resulting in high and stable blood drug concentrations. Its metabolite is ciprofloxacin, which also possesses strong antibacterial activity. Its bactericidal mechanism is direct and unique, directly acting on the bacterial nucleus by inhibiting bacterial DNA gyrase, leading to rapid bacterial death and making it less prone to developing drug resistance.
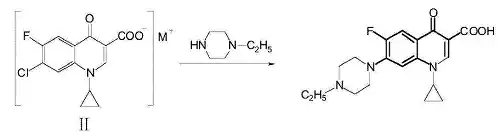
Preparation
A method for preparing enrofloxacin. The method involves: in a solvent, 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylate (II) undergoing a substitution reaction with N-ethylpiperazine at a certain temperature, followed by post-processing to obtain the target product enrofloxacin. The synthesis reaction is as follows:
The molar ratio of 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylate (II) to N-ethylpiperazine is 1:1 to 1:6; the substitution reaction can also include the addition of a Lewis acid catalyst, preferably AlCl3, ZnCl2, or SnCl4; the amount of Lewis acid added has a molar ratio to 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylate (II) of 0.01 to 0.6:1; the solvent is preferably an alcohol solvent, more preferably ethanol, isopropanol, or isoamyl alcohol, with the dosage being 2 to 20 ml of solvent per gram of 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylate (II); the reaction temperature is preferably 70 to 140°C; the specific preparation method for enrofloxacin is as follows: add 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-quinoline-3-carboxylate (II), N-ethylpiperazine, Lewis acid, and solvent to a reaction flask, control the temperature at 70 to 140°C, maintain the temperature for 8 to 10 hours until the N-ethylpiperazine substitution is complete, then cool and filter to obtain a solid. Dissolve the solid in dilute hydrochloric acid, adjust the pH to 7.2 to 7.3 with alkali, let it stand, and filter to obtain the crude product. Recrystallize from ethanol to obtain enrofloxacin. Enrofloxacin can be converted to enrofloxacin sodium by forming a sodium salt.
Specifications
Dosage and Administration
Oral Administration: Once daily dose per kilogram body weight: calves, lambs, piglets, dogs, cats: 2.5-5mg; poultry: 5-7.5mg; twice a day for 3-5 consecutive days.
Medicated Drinking Water: Per liter of water: poultry 50-75mg, for 3-5 consecutive days.
Intramuscular Injection: Once daily dose per kilogram body weight: cattle, sheep, pigs: 2.5mg; dogs, cats, rabbits, poultry: 2.5-5mg; once or twice a day for 2-3 consecutive days.
Applications
Clinically mainly used for infections caused by mycoplasma and various susceptible bacteria, such as Escherichia coli, Pseudomonas aeruginosa, Salmonella, Pasteurella, Haemophilus, Staphylococcus aureus, Staphylococcus, Streptococcus, Erysipelothrix rhusiopathiae, etc., such as chronic respiratory disease in chickens, swine enzootic pneumonia, swine white scours, piglet yellow scours, and swine edema disease.
Pharmacological Action
This product is a fluoroquinolone drug specifically for animals. It has a specific effect on mycoplasma and good antibacterial activity against various Gram-negative and Gram-positive bacteria, including Escherichia coli, Pseudomonas aeruginosa, Salmonella, Pasteurella, Proteus, Klebsiella, Haemophilus, Shigella, Aeromonas, Campylobacter, Staphylococcus aureus, Staphylococcus, Streptococcus, Corynebacterium pyogenes, Erysipelothrix rhusiopathiae, etc. Due to the unique antibacterial mechanism of fluoroquinolones, this product also has good efficacy against mycoplasma resistant to tylosin or tilmicosin, Staphylococcus aureus resistant to penicillin, and Pseudomonas aeruginosa resistant to gentamicin.
Pharmacokinetics
A study showed the enrofloxacin concentration values in the plasma of chickens after administration of enrofloxacin, enrofloxacin hydrochloride, and enrofloxacin sodium, as shown in the table:
| Time After Dosing (h) | Enrofloxacin (μg/mL) | Enrofloxacin Sodium (μg/mL) | Enrofloxacin Hydrochloride (μg/mL) |
| 0.083 | 0.35 ± 0.38* | 0.28 ± 0.23 | 0.21 ± 0.26 |
| 0.25 | 0.68 ± 0.65 | 0.73 ± 0.52 | 0.60 ± 0.91 |
| 0.5 | 1.20 ± 0.97 | 1.00 ± 0.71 | 0.96 ± 1.24 |
| 1 | 1.52 ± 0.99 | 1.18 ± 0.86 | 1.24 ± 1.13 |
| 2 | 1.95 ± 0.96 | 1.51 ± 0.84 | 1.72 ± 1.08 |
| 3 | 2.24 ± 0.68 | 1.76 ± 0.80 | 2.01 ± 0.93 |
| 4 | 2.29 ± 0.61 | 2.02 ± 0.57 | 2.43 ± 0.74 |
| 6 | 2.11 ± 0.51 | 1.84 ± 0.50 | 2.11 ± 0.33 |
| 8 | 1.62 ± 0.41 | 1.46 ± 0.40 | 1.85 ± 0.28 |
| 12 | 1.01 ± 0.35 | 1.02 ± 0.39 | 1.23 ± 0.21 |
| 24 | 0.53 ± 0.24 | 0.59 ± 0.30 | 0.77 ± 0.27 |
| 36 | 0.29 ± 0.11 | 0.41 ± 0.21 | 0.47 ± 0.18 |
*Note: The values represent the mean ± standard deviation of enrofloxacin concentration in μg/mL at each time point for each formulation. The asterisk in the first column is likely a formatting element and doesn’t carry specific meaning in the context of the data itself.
Pharmacokinetic parameters are shown in the table:
| Parameter | Unit | Enrofloxacin | Enrofloxacin Sodium | Enrofloxacin Hydrochloride |
| M | μg/mL | 3.23 ± 0.90 | 2.52 ± 0.86 | 3.15 ± 0.60 |
| Ka | /h | 1.24 ± 1.31 | 1.72 ± 1.63 | 1.33 ± 1.57 |
| K | /h | 0.09 ± 0.03 | 0.07 ± 0.03 | 0.07 ± 0.03 |
| t₁/₂Ka | h | 1.15 ± 0.99 | 0.76 ± 0.56 | 1.55 ± 1.72 |
| t₁/₂K | h | 8.39 ± 3.06 | 12.19 ± 7.46 | 10.95 ± 4.92 |
| t<0xE2><0x82><0x98>max | h | 3.54 ± 1.85 | 3.02 ± 1.77 | 4.65 ± 3.54 |
| Cmax | μg/mL | 2.39 ± 0.70 | 2.04 ± 0.66 | 2.37 ± 0.65 |
| AUC | mg/L·h | 36.75 ± 9.27 | 37.94 ± 14.88 | 46.60 ± 12.08 |
| F | % | 100 (reference) | 103.23 ± 40.50 | 126.80 ± 32.88 |
Explanation of Parameters:
- M: Likely represents some initial concentration or a derived parameter from the modeling process. Without further context, the exact meaning is unclear, but it’s a model-dependent parameter.
- Ka: Absorption rate constant. It describes how quickly the drug is absorbed into the bloodstream.
- K: Elimination rate constant. It describes how quickly the drug is removed from the bloodstream.
- t₁/₂Ka: Absorption half-life. It is the time it takes for half of the drug at the absorption site to be absorbed.
- t₁/₂K: Elimination half-life. It is the time it takes for the concentration of the drug in the plasma to reduce by half.
- tmax: Time to reach maximum concentration. It is the time at which the highest concentration of the drug is observed in the plasma.
- Cmax: Maximum concentration. It is the highest concentration of the drug achieved in the plasma.
- AUC: Area Under the Curve. It represents the total drug exposure over time in the plasma.
- F: Relative bioavailability. It compares the bioavailability of enrofloxacin sodium and enrofloxacin hydrochloride to that of enrofloxacin (set as the reference, 100%). Values greater than 100% indicate higher bioavailability.
The values in the table are presented as the mean ± standard deviation.
The experimental results showed that after oral administration of enrofloxacin, enrofloxacin sodium, and enrofloxacin hydrochloride solutions to chickens, the pharmacokinetic model in chickens was a first-order absorption one-compartment model, with high peak concentrations (Cmax of 2.39, 2.04, and 2.37 μg/mL, respectively), rapid absorption (absorption half-lives t1/2Ka of 1.15h, 0.76h, and 1.55h, respectively), and slow elimination (elimination half-lives t1/2K of 8.39h, 12.19h, and 10.95h, respectively). Using enrofloxacin as a control, enrofloxacin sodium and enrofloxacin hydrochloride were completely absorbed after oral administration, with high relative bioavailability of 103.2% and 126.8%, respectively.
Adverse Reactions
This product should not be mixed with kanamycin sulfate, gentamicin sulfate, chloramphenicol, etc., to avoid turbidity. Use with caution in animals with impaired renal function; adjust the dosage for severe renal or liver disease to prevent drug accumulation in the body.